I have repeatedly complained about how much I hate chemistry. But that’s changing. The more I learn about atoms and molecules, the more I learn about how they interact with each other, the more they blow my mind. It’s hard to hate a subject that is so consistently mind-blowing.
Recently, my mind was blown by something called electron delocalization (or electron resonance, if you prefer old school chemistry lingo). Basically, this is a fancy term for what happens inside a molecule when electrons go wild.
So within a molecule, there are certain positions that each atom is supposed to take, and they’re supposed to stay put (more or less). But electrons… electrons like to run around and play. Depending on molecular structure and the types of chemical bonds (pi bonds vs. sigma bonds), some molecules turn into awesome electron jungle gyms.
For example, here’s a benzene molecule.
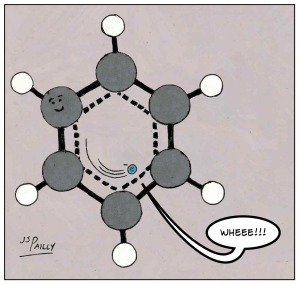
The ring shape of benzene is like a racetrack for electrons. Electrons can just run round and round to their subatomic hearts’ content. As a result benzene molecules—and other, more complicated molecules that incorporate benzene rings—are very stable. Extremely stable. This might seem counterintuitive, but the more “electron delocalization” occurs in a molecule, the more stable a molecule tends to become.
If you have even a passing familiarity with quantum physics, you might guess what’s really happening here. Electrons don’t merely run around inside a molecule; electrons exist simultaneously in multiple locations inside that molecule. And the more spread out electrons are allowed to be, the more they can help tie the molecule together.
But while electron delocalization is great fun for electrons, and while it helps stabilize a molecule overall, certain parts of a molecule can feel a little left out. Certain protons (hydrogen ions) in particular will feel neglected and lonely. In the next edition of Molecular Mondays, we’ll find out what happens to them.
* * *
Today’s post is part of a special series here on Planet Pailly called Molecular Mondays. Every other Monday, I struggle valiantly to understand and explain some concept in the field of chemistry. Please note: I suck at chemistry, but I’m trying to learn. If I made a mistake, please, please, please let me know so I can get better.
One reason the molecule is more stable when you have electron delocalization is because the electrons find themselves in lower energy states, which makes them happy. And any reaction which might change this and forces the electrons into higher energies is going to be met with armed resistance. They like being low energy. Just like me, I suppose.
LikeLiked by 1 person
Makes sense to me. That’s basically thermodynamics at work, right? Everything is trying to get to the lowest energy state possible and stay there.
LikeLike