
Welcome to Molecular Monday! On the first Monday of the month, we take a closer look at the atoms and molecules that make up our physical universe. Today, we’re looking at:
CPK Coloring
When I first introduced this Molecular Monday series, I knew I’d be drawing a lot of atoms and molecules, but I wasn’t sure if there was a right way or a wrong way to draw them. For starters, I wasn’t even sure what colors I should use.
Atoms and molecules do not really have colors, in the sense that they’re too small for visible light to reflect off them. At one point, I wondered if I should color them based on their spectroscopic signatures, but that line of research got complicated really fast.
Eventually I discovered that chemists have a (mostly) standardized color-coding system for modeling the atoms in a molecule. It’s called CPK coloring, in honor of Robert Corey, Linus Pauling, and Walter Koltun. Apparently Corey and Pauling created this system in the 1950’s, and Koltun improved it by adding more colors in the 1960’s (improving things by adding more colors is basically what the 60’s were all about).
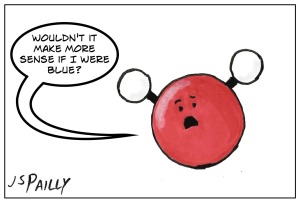
So following the CPK coloring scheme, hydrogen atoms are white, and oxygen atoms are red. (Example: water molecule, H2O.)
Nitrogen atoms are blue. (Example: molecular nitrogen, N2.)

Sulfur atoms are yellow. (Example: hydrogen sulfide molecule, H2S.)

And carbon atoms are either black or grey. I draw them in grey because otherwise you couldn’t see their little smiley faces. (Example: benzene molecule, C6H6.)

Personally, I think it would make more sense to switch the colors of oxygen and nitrogen. That way, water molecules would have blue in them, rather than bright red. But otherwise, CPK coloring is a pretty good system.
Typically green is assigned to either chlorine or fluorine, or sometimes both. Beyond that, modern chemists seem to have strayed from the original psychedelic system Koltun invented. I guess the rarer an element is, the less we worry about sticking to a standardized color code.
For my purposes, that hasn’t been a problem. Almost every molecule I write about on this blog is composed of carbon, hydrogen, and maybe oxygen and/or nitrogen. Occasionally sulfur gets into the mix, but that’s basically it.
For next month’s Molecular Monday post, I think we’ll continue looking at some of the other issues involved with drawing molecules. I settled on ball-and-stick models, but that’s not the only way to do things.
I suspect oxygen got red because of rust. For some reason, hydrogen seems like it should be red and oxygen white to me. I’m good with nitrogen getting blue and carbon getting grey or black.
I have no idea why I have these preferences.
LikeLiked by 1 person
I could get behind hydrogen being red. It’s strongest emission line is in the red part of the spectrum.
LikeLiked by 1 person
The rust idea makes sense. I think blue makes sense for Nitrogen too, since the sky is blue and the atmosphere is mostly Nitrogen.
LikeLiked by 2 people
I get that. Makes sense. It just bothers me a little that water molecules don’t have any blue in them.
LikeLike